Oxidation of Silverware Caused by Reaction with Oxygen Gas and/or Hydrogen Sulfide Gas?
 |
| Silver(I) Oxide |
 |
| Tarnished Metal |
| Silver Sulfide |
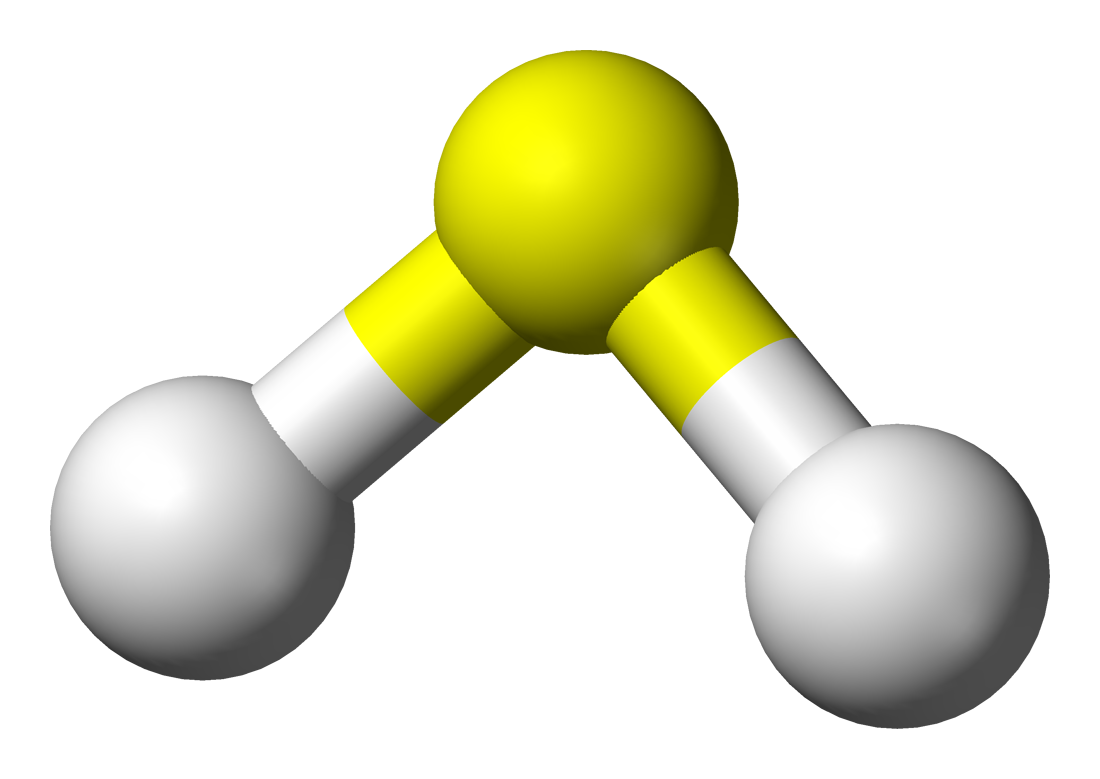 |
| Sulfur Dioxide |
A simplified chemical reaction of oxidizing silver is [2]
4 Ag(s) + 2 H2S(g) + O2(g)
However tarnishing is a multistep process. This reaction "is a short-hand or end-result of many transformations. " The detailed reactions are given below.
Reduction and Oxidation Explanation
In this reaction silver goes from an oxidation state of zero in the reactants to +1, the oxidation state in the products (oxygen has a -2 oxidation state in water). Hydrogen remains the same at +1 and sulfur remains at an oxidation state of -2 on both sides of the equation. Oxygen is in its elemental form and has an oxidation state of zero on the reactants side and -2 on the products side. Silver is oxidized (reducing agent); its oxidation number increases and oxygen is reduced (oxidizing agent).Can Hydrogen oxidize Silver?
Another alternative reaction given does not include oxygen. If you were to look at a table of electromotive force you would know that this could not happen the EMF would be -0.8Volts.2 Ag(s) + H2S(g) ---> Ag2S(s) + H2(g)
In this reaction hydrogen is reduced from +1 to zero and silver is oxidized from zero to +1.The oxidation state of sulfur remains unchanged.
Multistep Tarnishing of Silver
Corrostion and tarnishing are multistep reactions. Even the formation of rust is not a simple combination of iron and oxygen. Water is necessary for rust to form. Upon closer research of articles on the internet there is one website that talks about the multistep mechanism of silver tarnishing.8Ag + 4HS- <---> 4Ag2S + 2H2 + 4e-
02 + 2H2O + 4e- <---> 4OH-
What would be the sum of the EMF of these reactions?
Ag ---> Ag+ + e- EMF = -0.799V
2H+ --->2e-+H2 EMF = 0V
02 + 2H2O + 4e- <---> 4OH- EMF = 0.4V
This reaction would not be spontaneous because the sum of the EMF's would be negative (-0.799+0.4=0.399V)
If the reaction were under acidic conditions the EMF would be large enough to tarnish the silver:
02(g) + 4H+(aq) + 4e- <---> 2H2O(l) EMF = 1.23V
-0.799+1.23= 0.431V
Why Doens't Silver Oxide form When Silver is Tarnished?
Activity Series
Alkali and alkaline earth metals are the most active, most easily oxidized because their electron can be easily ionized. However transition metals and "noble"metals such as silver hold on to their electrons more tightly. Oxidation, therefore has a relationship to ionization energy. The lower the ionization energy the easier it is to oxidize an element.Silver is a Less Reactive Noble Metal
One reason why silver does not form silver oxide when it reacts with air is that silver is not as reactive as some other metals, where reactivity is defined as the ability to form a positive ion. Reactive metals tend to have 1 valence electron in the s shell or the p shell, valence electrons and ionization energies (energy to eject an electron).So why does silver react with sulfide ions in solution vs. hydroxide? When silver gets oxidized sulfur is probably the first thing it sees and so it reacts with it. To get it to react with oxygen and form an oxide it would take converting OH- or water to oxide (O2−). First the silver might have to form Ag(OH) in the steps similar to how the iron in steel becomes rust.
Solubility of Silver Sulfide and Silver Hydroxide in Solution?
The solubility product constant for silver is 6x 10-51, which means that not much Ag2S does not like to form the ions Ag+ and S-, whereas Ag(OH) is
1.52 x 10 -8. Silver hydroxide is much more soluble in water. It is therefore most likely that silver does not form silver oxide because it is more soluble in water and solid Ag(OH) is less likely to form than solid Ag2S in solution.
A Green Way to Remove Silver Sulfide Tarnish
A common way to remove the silver sulfide tarnish is to put the silverware in a solution of baking soda, warm water and aluminum. Aluminum is very reactive and will form aluminum oxide (Al2O3), a white powder. This is why we add baking soda. It creates a basic solution that helps to break through the outer aluminum oxide layer. The chemical equation for baking soda in water is [4]
NaHCO3 (baking soda) + H2O →
H2CO3 + Na+ + OH-
The reaction of silver sulfide with Aluminum can be expressed in the following reaction(s):
3 Ag2S(s) + 2 Al(s) ---> 6 Ag(s) + Al2S3(s)
3Ag2S(aq) + 2Al(s) → 6Ag(s) + 2Al+3(aq)+ 3S- 2(aq)
or including the water3Ag2S(aq)+ 2Al(s) + 3H2O(l) → 6Ag(s) +Al2O3 (aq) + 3H2S(g)
Breaking the Reaction into Two parts:
OXIDATION: 2 Al(s) + 6 OH– (aq) –––> Al2O3(s) + 3 H2O (l) + 6 e–
REDUCTION: Ag2S(s) + 2 H2O (l) + 2 e– –––> 2 Ag(s) + H2S (aq) + 2 OH– (aq)
3 Ag2S(s) + 2 Al(s) + 3 H2O (l) –––> 6 Ag(s) + 3 H2S (aq) + Al2O3(s)
Aluminum Sulfide is a white powder.
| Aluminum Sulfide |